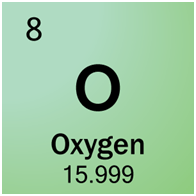Mary had a shorn a little lamb
Its fleece was filthy grey.
Mary had to scrub it clean.
For a sock on Mom’s birthday.
She took it to an automat.
But wondered what to add.
Sodium carbonate will do the trick.
But adding too much was bad.
So, Mary had to figure out
How many atomic grams to use.
To do this she drew up its molar mass
And started to deeply muse.
Before we get started, or if you need additional help, come to Studygate to hire an online chemistry tutor. Let us learn here to find molar mass, specially with an example molar mass of Na2Co3. Molar mass is defined as its “gram-formula-mass.” In other words, molar mass is how much one mole of the substance weighs in terms of “atomic mass.” A mole is a number: 6.02×1023. Just like a dozen is 12, one mole is 602000000000000000000000. Scientists use moles as a base number so they can compare groups of atoms. Just as you can compare one carbon atom to one helium atom, you can compare one mole of carbon to one mole of helium. This is interesting because chemistry affects who we are as humans and even influences the nutrition of the foods we eat.
*to abbreviate “mole,” just shorten it to “mol.”
The larger the atom is, the heavier it weighs. This makes sense, right? Well, the periodic table is organized from smallest to largest, in which the number of protons and neutrons largely determine the weight (Atoms have electrons, but they are so small they weigh “nothing”).
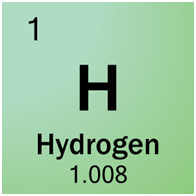
For example, in the top left corner, Hydrogen has one proton, one electron, and no neutrons. So it’s atomic number is One (for one proton) and its atomic weight is One (for one proton and one neutron)
One mole of hydrogen atoms will be its atomic weight. One mole of hydrogen atoms weighs One gram/mol.
The next one is helium, which has two protons and two neutrons. This means the atomic number is Two (for two protons) but the atomic weight of the atom is FOUR (two protons and two neutrons).
One mole of helium atoms weighs Four gram/mol.
**Here’s a quick tip, in the more common elements near the top of the chart, an atom’s protons and neutrons are “ideally” the same. So, the atomic weight is simply doubled the atomic number. Pretty easy, huh?
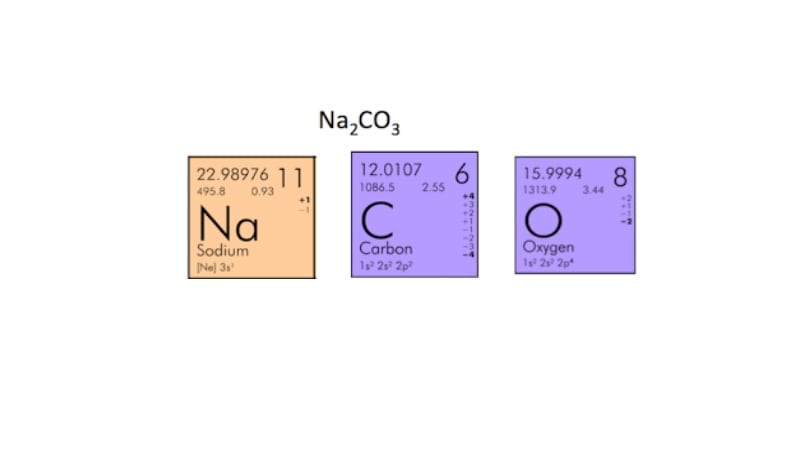
Na2Co3 is made up of two sodium atoms, one carbon atom, and three oxygen atoms. We know the number of each atom because the number of atoms per element is listed right after the symbol.
Let’s look at each atom:
2 Na
1 C
3 O
Then, just add up the atomic weights of each:
(2 x 22.990) + (12.011) + (3 x 15.999) =
44.980 + 12.011 + 47.997 =
105.987 grams/mol
Looks like Little Mary has her work cut out for her!